Doctor Healthy Search

Custom Search

FULL HLA TRANSPLANT MATCHING LEADS TO MORE SKIN CANCER
Rates of de novo skin cancer after lung or heart transplantation are higher when donor and recipient HLA antigens are well matched, a large retrospective study suggests.
Lung and heart recipients with HLA mismatches "actually had less skin cancer than those with a closer match," Dr. Sarah T. Arron told Reuters Health by email. "This suggests that the immune system of patients with mismatches may still be able to recognize and fight off skin cancer cells despite the high level of immunosuppression required to protect the transplanted organ."
"Internists and dermatologists who treat organ transplant recipients should be aware that the risk of skin cancer may be higher in patients who underwent thoracic transplant and received a well-matched organ," Dr. Arron of the University of California, San Francisco, and colleagues write in a January 23 online paper in JAMA Dermatology.
They note that immunosuppressive regimens that reduce the risk of graft rejection increase the risk of cancer, a major source of morbidity and mortality in solid-organ transplant recipients.
Molecular mechanisms play an important role in the defense against the development of cancer, but "data on the association between HLA antigen mismatch and skin cancer incidence are limited, and kidney transplant recipients have been the focus," they add.
To investigate further, the team examined data on more than 10,600 recipients of lungs, hearts, pancreata, livers, and kidneys between 2003 and 2008. The study, which was conducted between 2016 and 2017, matched participants to skin cancer outcomes by medical record review.
"A greater number of mismatched alleles, as identified through the standard antigen mismatch and broad specificity mismatch methods, was associated with a protective effect," the researchers report.
In particular, a 7% to 8% reduction in skin cancer risk was found for each additional mismatched allele. Subgroup analysis showed that HLA antigen mismatch led to significant protection in lung recipients (adjusted hazard ratio, 0.70 ) and heart recipients (aHR, 0.75), but not for those who received a liver, kidney, or pancreas. The degree of HLA-DR mismatch was the most statistically significant for skin cancer risk (aHR, 0.85).
"The aHRs for squamous cell carcinoma and melanoma were similar to those for any skin cancer, but the HR was not statistically significant for melanoma because of the smaller number of events," the researchers report.
In light of these findings, they conclude that "well-matched heart and lung transplant recipients may have a higher risk of skin cancer after transplant." And, added Dr. Arron, "Future studies are still needed to learn how different immunosuppressive drugs impact this finding."
Commenting by email, Dr. Mark Faries, Co-Director of the Cutaneous Oncology Program and Professor of Surgery at Cedars-Sinai Medical Center, Los Angeles, told Reuters Health, "The immediate implication is that for heart or lung transplant patients, the risk for skin cancers is going to be highest for patients with the best matches. Over the long term, this research may lead to identification of the mechanisms at work for the patients with poor matches that protect them from skin cancers, and perhaps new therapies."
OBESITY RELATED CANCERS ON THE RISE
In the United States from 1995 to 2014, the incidence of six of 12 obesity-related malignancies increased among "young" adults (25-49 years), according to a new observational study.
However, the incidences for these cancers — except for colorectal cancer —also rose in older adults (50 years or older), acknowledge the authors, led by Hyuna Sung, PhD, cancer epidemiologist and principal scientist, Surveillance and Health Services Research Program at the American Cancer Society. The study was published onlinetoday in The Lancet Public Health.
But the young adults, who were the focus of the study, had larger annual percentage increases than the older adults.
In young adults, the six obesity-related cancers that increased in incidence in were multiple myeloma, colorectal, uterine corpus, gallbladder, kidney, and pancreatic cancer.
On the other hand, the six obesity-related cancers that did not increase in young people were breast, esophageal, gastric cardia, liver and intrahepatic bile duct, thyroid, and ovarian.
Despite the findings, the study is not evidence of a causal relationship between obesity and cancer.
Furthermore, an expert not involved with the study questioned the concept of "obesity-related" cancers.
"The obesity–cancer story is far from clear and while the authors selected cancers that might be obesity related, they also might be related to other factors not considered that may be changing over time but that were not examined," Ruth Etzioni, PhD, a biostatistician at Fred Hutchinson Cancer Research Center in Seattle, told Medscape Medical News.
"I worry about inflammatory articles like this one misinforming the public," she added.
The obesity–cancer story is far from clear.
Dr Ruth Etzioni
Nevertheless, senior author Ahmedin Jemal, DVM, PhD, vice president of the Surveillance and Health Services Research Program at the American Cancer Society, sounded an alarm about the results.
"Our findings expose a recent change that could serve as a warning of an increased burden of obesity-related cancers to come in older adults," he said in a press statement. "Most cancers occur in older adults, which means that as the young people in our study age, the burden of obesity-related cancer cases and deaths are likely to increase even more."
Jemal and coauthors called for increased obesity screening in young adults.
In an accompanying editorial, Catherine Marinac, PhD, and Brenda Birmann, ScD, of Harvard University in Boston, Massachusetts, say that it is "plausible" as well as "provocative" that obesity is driving the reported results. But, they add, the investigators' interpretation of that relationship is "speculative."
Furthermore, the editorialists write that they would have liked to have heard the study authors' thoughts about why only some obesity-related cancers were on the rise in young adults — and not all 12.
Both sets of experts call for further research to uncover the exposures responsible for these "emerging trends."
For example, over the study period, the average annual change for pancreatic cancer was equal or less than 1% in people ages 40 to 84, 1.3% in those ages 35 to 39, and 2.5% in 30 to 34-year-olds. In the youngest age group (ages 25-29), it was 4.3%.
The investigators did not look at solely obesity-related cancers.
The researchers also reviewed incidence data on 18 other (non–obesity-related) cancers. And the findings were telling, they suggested: "…the incidence increased in successive younger generations for only two of the 18 additional cancers, and decreased for about half of the remaining cancer types."
In their study discussion section, the authors comment extensively about obesity in the United States and suggest their new findings may be related to recent trends showing increases in body weight.
"These [cancer incidence] trends might have been influenced by the rapid rise in overweight or obesity prevalence in the USA. Between 1980 and 2014, overweight or obesity prevalence in the USA increased by more than 100% (from 14.7% to 33.4%) among children and adolescents and by 60% among adults aged 20-74 years (from 48.5% to 78.2%)," they write.
Lead author Sung commented at length about food quality as a possible contributor to the newly found trends: "Obesity is associated with health conditions that can contribute to the risk of cancer. For example, diabetes, gallstones, inflammatory bowel disease, and poor diet can all increase the burden of cancer," she said. "The quality of the American diet also has worsened in recent decades. More than half of adults who were 20 to 49 years old between 2010 to 2012 reported poor dietary habits, such as eating little fruits, vegetables, whole grains, fish, and shellfish at the same time as eating too much salt, fast food, and sugary drinks."
The study was funded by the American Cancer Society and the National Cancer Institute. The study authors, editorialists, and Etzioni have disclosed no relevant financial relationships.
Eighteen Other Cancers Examined
Notably, the study authors also report that, among the six cancers on the rise in the young adults, there was a steeper increase in progressively younger ages (P wald < .05).
ACTIVE SURVEILLANCE FOR THYROID CANCER
Patients with thyroid cancer who choose to have active surveillance instead of surgery report a similar psychological burden of worrying about their cancer as patients who undergo thyroidectomy. However, those concerns lessen over time and most ultimately express satisfaction with their treatment choice, according to a large new study of patients with thyroid cancer in Japan.
"It is reassuring to find that the burden of concern, such as worry, is similar between patients who are treated and those who are surveilled," say Louise Davies, MD, of the Geisel School of Medicine at Dartmouth, in Hanover, New Hampshire, and colleagues, in their article published online January 31 in JAMA Otolaryngology–Head & Neck Surgery.
"These findings suggest that the possibility of cancer worry should not be viewed as prohibitive to successful active surveillance in thyroid cancer," they write.
Nevertheless, "the observation that one third of patients with thyroid cancer receiving long-term active surveillance harbor worry that affects their mood deserves attention," they add, noting, "the role of the physician, and medical care more broadly, is to relieve suffering."
In an accompanying commentary, Anna M. Sawka, MD, PhD and David P. Goldstein, MD, of the University of Toronto, Ontario, Canada, suggest the findings are a wake-up call for clinicians to be aware of the potential concerns patients may have about their diagnosis, regardless of whether they choose active surveillance or surgery.
"The important lessons for healthcare practitioners are not to underestimate the importance of the diagnosis, treatment, and follow-up of thyroid cancer and to fully address the supportive care needs of this population, irrespective of treatment choice."
Data on Active Surveillance in Small Papillary Thyroid Cancers Lacking
Small thyroid cancers account for 50% or more of detected cases, and with such cancers often failing to become symptomatic over a person's lifetime, patients may be offered active surveillance with periodic imaging or testing instead of immediate surgical intervention.
Some have speculated, however, that the psychological concern of the cancer possibly growing could be greater than that with more definitive treatment, but data on this have been lacking.
To take a closer look, Davies and coauthors conducted a survey of patients at Kuma Hospital, in Kobe, Japan, which is the site of the world's largest cohort of patients undergoing active surveillance for papillary thyroid microcarcinoma.
Of 215 patients who completed the survey, 195 were women and 20 were men.
Overall, among all respondents, 37% reported that their worry about their cancer occurred sometimes (as opposed to rarely for 42% or not at all for 21%), and 32% said the worry affected their mood either somewhat or a lot (compared with "a little" for 44% and not at all for the remaining 24%).
And 14% of patients said the worry affected their ability to carry out daily activities somewhat or a lot.
Among the leading causes of patients' worry were fear of the cancer spreading, the possible need for later surgical intervention, and difficulty interpreting bodily experiences in the general location of the cancer.
However, as many as 60% reported a decline in their level of worry from the time they first learned of the cancer.
By 3 years after diagnosis, the proportion of participants who said they were not at all worried about the cancer increased from 14% to 25%.
Despite the levels of concern, the vast majority of patients said they agreed or strongly agreed that their decision to have active surveillance matched their personal values, and 83% agreed or strongly agreed that the active surveillance choice was the best decision for them personally.
Concerns Similar to Other Low-Risk Cancers, Provide Support
"To our knowledge, this study is the first report about worry among patients with thyroid cancer on active surveillance," the authors note.
And the findings illustrate that examining comparative data from other cancers with similar diagnosis "is informative," they observe.
For example, there has been a study of active surveillance of prostate cancer since the 1980s, which has similarly shown that quality of life and mental health measures were generally no worse in patients undergoing active surveillance compared with those who chose active management.
The new study likewise underscores that "efforts to improve the survivorship experience of this patient population should include both those whose first management choice is surgical intervention and those whose first management choice is surveillance," the authors stress.
Those efforts could include offers of patient support groups, referral to psychological care, or advice in handling concerns about cancer recurrence, they indicate.
"Currently, most patients do not receive these services," they emphasize.
In their commentary, Sawka and Goldstein note one important limitation of this study is that they did not compare those undergoing active surveillance with a surgical comparison group at Kuma Hospital.
But there are comparative data from other studies, they note.
"Indirect comparisons of these findings to published studies of patients with papillary thyroid cancer [PTC] who had surgical treatment from other institutions and countries suggest that cancer-related worry may not necessarily be worsened by active surveillance," they explain.
That being said, more research is needed to validate the findings in other settings to understand its generalizability.
"This important research highlights the critical need for future prospective long-term outcome research, comparing not only the oncologic outcomes but also the experiences of patients with low-risk PTC under active surveillance with those treated by thyroidectomy," they write.
As previously reported by Medscape Medical News, the approach of active surveillance for low-risk PTC first gained recognition at Kuma Hospital; however, many are still reluctant to adopt the approach outside of Japan.
The study was funded in part by the US Department of Veterans Affairs, Dartmouth Institute for Health Policy & Clinical Practice, and the National Institutes of Health/National Cancer Institute.
TACHYCARDIA INCREASES CANCER MORTALITY RISK
Among cancer patients who have tachycardia but who have not had a medical condition that is associated with tachycardia, such as pulmonary disease or thyroid disease, overall mortality risk is markedly increased. That increased risk may reflect the extra strain placed on the body by their cancer, say US researchers.
Tochi M. Okwuosa, DO, director of the cardio-oncology program at Rush University Medical Center, Chicago, Illinois, studied more than 620 cancer patients, of whom 50 were found to have explained sinus tachycardia.
After taking into account numerous potential confounding factors, the investigators found that for cancer patients with tachycardia, overall mortality risk was approximately three times that of other cancer patients.
The results were presented at the Advancing the Cardiovascular Care of the Oncology Patient conference on January 25.
Study coauthor Mohamad Hemu, MD, also from Rush University Medical Center, said in a statment: "Tachycardia is a secondary process to an underlying disease and reflective of significant multisystem organ stress and disease in cancer patients.
"As a result, the most important initial step is to figure out what is causing the tachycardia," he continued. "Reversible causes like dehydration and infections should be ruled out. Additionally, cardiopulmonary processes such as pulmonary embolism and other arrhythmias must be taken into consideration.
"Once these and all other causes of tachycardia are ruled out, then it is more likely that sinus tachycardia is a marker of poorer prognosis in these patients," Hemu commented.
While calling for more studies, Okwuosa said that their study "shows that tachycardia is a strong prognosticator regardless of cancer type."
Tachycardia is a strong prognosticator regardless of cancer type. Dr Tochi Okwuosa
"That's why it is critically important to be co-managing both cancer and heart conditions to ensure patients receive the most effective treatment possible."
Speaking to Medscape Medical News, Okwuosa explained that their hypothesis is that tachycardia reflects the metabolic strain placed on the patient's system by the cancer.
As an analogy, she said that when healthy people run, their heart rate goes up, and they in effect become tachycardic.
"Generally what happens is your body speeds up your heart rate in order to supply more blood to your tissues when you're running, because your tissues need that blood flow," she said.
"Well, in somebody who's very sick and they have a systemic issue going on that's affecting their entire body, such as cancer, I feel the sicker they are, the more their cells are affected, the more the rest of the body is affected, and so the higher the demand to try to meet the metabolic needs of the cancer."
Okwuosa said that while they await the results of further studies on the effectiveness of beta blockers in treating unexplained tachycardia in cancer patients, "one of the ultimate questions is, What kind of advice do you have for patients that are tachycardic and are going through cancer treatment?
"In all honesty, it's hard to figure out what's the right answer. Do you tell the patient this suggests that you're really sick and this suggests that there's a high chance that you're going to die?
"No, I think that most of us would not do that, especially since this is new data that's coming out," she said.
On the other hand, Okwuosa suggested that, although cancer patients may not be able to perform the recommended 150 minutes of exercise per week, they should try to walk or cycle as much as possible to condition their bodies.
"We know that, in normal patients, the more you exercise, the slower the heart rate, because there's increased vagal tone, but the kind of advice to give cancer patients becomes more difficult, just because they're really sick," she said.
"But we still encourage them to condition themselves," she added.
Approached for comment, Gordon F. Tomaselli, MD, Albert Einstein College of Medicine, New York City, said that, once the major causes of tachycardia have been ruled out, "I really do think you need to extensively look at the heart to make sure that there's not something that's a potential complication that in fact may get worse and at the very least be treated."
He added, "It's not at all surprising to me...that unexplained tachycardia is an adverse predictor of outcome, and there may be many reasons for it."
Tomaselli emphasized that it is "increasingly important" that when a cancer patient is about to start treatment, "you make sure that you understand the underlying state of their heart and vasculature.
"Nobody will thank you if you get successful treatment for a cancer but it leaves you with really significant cardiovascular disease that's going to limit your life expectancy and, more importantly, may on a day-to-day basis make the morbidity of the treatment pretty high," he added.
Study Details
Okwuosa and colleagues conducted a retrospective case-control analysis of 622 patients with lung cancer, leukemia, lymphoma, or multiple myeloma who underwent treatment from 2008 to 2016.
Tachycardia was defined as a heart rate ≥100 bpm at three or more clinical visits within 1 year of the cancer diagnosis.
After excluding patients with a history of pulmonary embolism, thyroid
For the 622 cancer patients who were included in the study, the mean age was 70 years, 60.5% were women, and 76.4% were white.
Of all cancer patients, 69.4% had American Joint Committee on Cancer stage IV disease, and 43.0% had lung cancer.
After adjusting for age, race, albumin, hemoglobin
< .01).
In a second model that adjusted for age, race, albumin, beta blockers, aspirin, coronary artery disease, stroke, diabetes, smoking, radiation, and athracyclines, tachycardia was associated with overall mortality at a hazard ratio of 2.8 (P < .001).
Future Plans
The researchers are planning to conduct an analysis to compare the outcomes of the tachycardia patients who received beta blockers and those who did not, although Okwuosa pointed out that the number of patients in these groups is relatively small.
She said: "We have just a few, 50 something, cancer patients all together, so this would be something that would be good in a larger study to see if we use beta blockers, that makes a difference in terms of mortality in the patients that are tachycardic."
Okwuosa continued: "That's something that's in the pipeline, something that we're still analyzing and something that we're thinking of obtaining funding for to study in a prospective manner in a randomized controlled study."
Tomaselli agreed that if the tachycardia "is a reflection of relatively minor but existing heart muscle weakness," owing, for example, to cancer treatment, "treatment with beta blockers might in fact improve outcomes."
He added: "This cohort is complicated by the fact that that they've had a primary malignancy, and a primary malignancy that may be the more important driver of overall mortality and outcome at the end of the day, but if in fact it's related to the heart, I think beta blockers are probably a good treatment option."
No funding for the study has been reported. The investigators have disclosed no relevant financial relationships.
Advancing the Cardiovascular Care of the Oncology Patient: Abstract 21. Presented January 25, 2019.
CONDITIONS TREATED WITH CANNABIS
Many conditions that are considered qualifying conditions for cannabis use under state laws have no or insufficient evidence of efficacy, and some have evidence of inefficacy, according to an analysis published online February 4 in Health Affairs.
Such conditions include dementia, glaucoma, hepatitis C, cachexia, irritable bowel disease, and numerous others, write Kevin F. Boehnke, PhD, a research investigator in anesthesiology at the University of Michigan in Ann Arbor, and colleagues.
However, the authors note that 85.5% of all patient-reported qualifying conditions had either substantial or conclusive evidence of therapeutic efficacy, based on a 2017 report by the National Academies of Sciences, Engineering, and Medicine (NASEM).
Chemotherapy-induced nausea and vomiting was linked with conclusive evidence of efficacy in the NASEM report. Multiple sclerosis and chronic pain had substantial evidence of efficacy.
Despite the uneven level of evidence for efficacy, medical marijuana is allowed under some states' laws for these conditions. Patients must have a physician certify that they have a qualifying condition to obtain a state license to use cannabis.
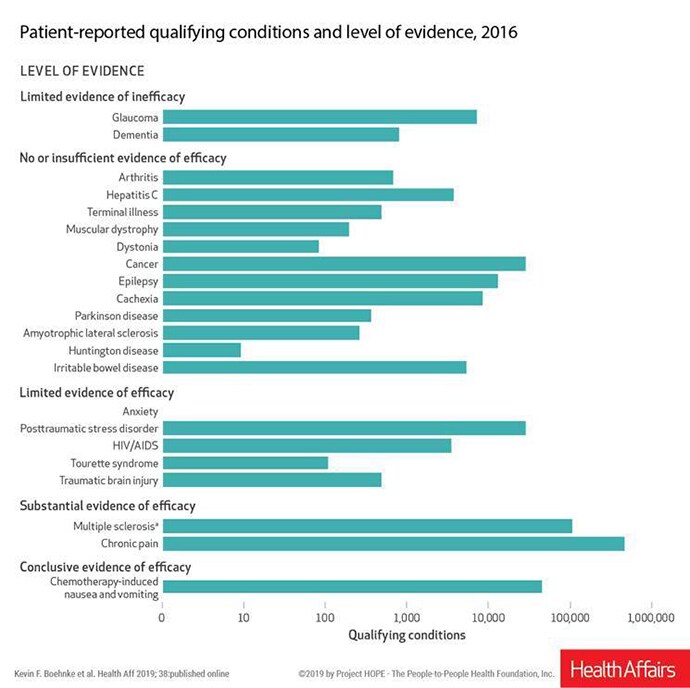
Boehnke told Medscape Medical News that the 85.5% figure sends a message to policymakers and physicians that these laws "are not just pothead laws" and that for the most part medical cannabis is being used as it was intended.
It also suggests that "patients and physicians should have an open dialogue about how the patient is planning to use cannabis and have check-ins about whether it's working or not," he said.
Chronic Pain Tops List of Qualifying Conditions
Chronic pain continues to be the most common qualifying condition reported by patients who use medical cannabis (64.9% in 2016), according to the article.
The authors had hypothesized that the conditions for which there was little to no evidence of efficacy or possible evidence of harm would have the lowest numbers of qualifying-condition claims, but that wasn't always the case in their analysis.
For example, Boehnke said, evidence does not support the use of medical marijuana to treat people with glaucoma or dementia, according to NASEM, which put both conditions in the "limited evidence of inefficacy" category. Boehnke said that means there is some evidence cannabis does not help or could cause harm. However, physicians are recommending it in a substantial number of instances — more often than for some conditions for which there is some evidence of efficacy.
So why is medical marijuana recommended for conditions for which there is little to no evidence that it is effective?
There are many answers for that, Boehnke said.
One has to do with the "Wild West" of cannabis, he said. Because there is no federal regulation, states decide whether to legalize it and then decide their own policies.
There also may be a financial incentive for some physicians, Boehnke said. He noted that in Michigan, for example, many medical marijuana clinics have popped up since the state allowed medical marijuana in 2008 and physicians in these clinics can make a recommendation in minutes.
"There is a financial incentive for some physicians to recommend the licenses for patients because they can get a per-appointment fee. There are some physicians who recommend licenses for hundreds or thousands of patients," he said.
Another is a lack of overall evidence, as research on the schedule 1 substance has been very limited. Also, when trials are conducted, they often involve a patient taking a certain amount of one type of cannabis a set number of times per day in a certain way of consumption, which is not how patients use the drug in real life. So confidence in the evidence may be understandably low.
Also, evidence that cannabis is effective or ineffective for a condition can change. Boehnke notes that last year a purified formulation of cannabidiol (CBD) (Epidiolex oral solution, GW Pharmaceuticals) was approved by the US Food and Drug Administration in 2018 to treat seizures in rare forms of epilepsy in children older than aged 2 years, as reported by Medscape Medical News. The 2017 NASEM report lists epilepsy as a condition for which there is little to no evidence of efficacy.
Registries, Research Lacking
The authors examined state registry data and reports available as of April 2018, but they acknowledge registry data have limitations.
Currently, 33 states and the District of Columbia have legalized cannabis for medical use, but use of registries varies greatly. Some states have minimal or no information in voluntary registries, other states collect substantial data and publish it in reports.
"As medical cannabis use continues to increase, creating a nationwide patient registry would facilitate better understanding of trends in use and of its potential effectiveness," the authors write.
Data consistency could also influence insurance coverage and need for more federal oversight, they note.
Pointing to the 85.5% of conditions for which there is substantial or conclusive evidence of efficacy of cannabis, the authors conclude that "state and federal policymakers should begin evaluating evidence-based ways for safely integrating cannabis research and products into the healthcare system."
Boehnke has reported receiving support from the National Institute of Dental and Craniofacial Research. Other authors have reported receiving support from the National Center for Advancing Translational Sciences and consulting for Pfizer, Eli Lilly, Tonix Pharmaceuticals, Aptinyx, Regeneron, IMC, and Intec.
PROGRESS IN RARE CANCER ADVANCE OF THE YEAR
Advances in the treatment of rare cancers have been chosen as the clinical cancer advances of the year by the American Society of Clinical Oncology (ASCO).
ASCO's annual report on progress against cancer, issued for the 14th consecutive year, was published online January 31 in the Journal of Clinical Oncology. The report covers the period from November 2017 through October 2018.
The report highlights five studies that show "significant steps forward" in the treatment of rare cancers, which together account for about 20% of all cancers diagnosed in the United States.
The five advances in rare cancers highlighted in the report were as follows:
- Targeted therapy for anaplastic thyroid carcinomas (ATCs). This is a rare type of thyroid cancer, accounting for fewer than 2% of cases. It is associated with a worse prognosis than more common forms of thyroid cancer. In 2018, the US Food and Drug Administration approved a new treatment for ATC for the first time in 50 years. The agent, for use in the treatment of BRAF-mutated ATC, consists of a combination of the targeted therapies the BRAF inhibitor dabrafenib (Tafinlar, Novartis) and the MEK inhibitor trametinib(Mekinist, Novartis). The approval was based on results from a single-arm phase 2 trial in 16 patients with BRAF-mutated tumors, all of whom had received prior radiotherapy, surgery, and/or chemotherapy. Of these patients, 69% responded to the drug combination, and at the time of publication, nearly half (seven patients) had experienced ongoing responses (J Clin Oncol. 2018;36:7-13).
- Sorafenib benefit in desmoid tumor. This rare type of sarcoma, which can regress spontaneously, was previously treated with off-label products. In what was hailed as a landmark study, investigators conducted a global, phase 3 trial in 87 patients with unresectable progressive desmoid tumors. The rate for 2-year progression-free survival (PFS) was 87% among patients who received the tyrosine kinase inhibitor sorafenib (Nexavar, Bayer), compared to 43% for those taking placebo (N Engl J Med. 2018;379:2417-2428).
- Radiolabeled drug benefit in midgut neuroendocrine tumors (NETs). This is a rare cancer, thought to affect fewer than 3 per 100,000 people annually. It is usually treated with somatostatin or octreotide(Sandostatin, Novartis), a synthetic long-lasting form of somatostatin. The advance is with the product lutetium dotatate Lu-177 (Lutathera, Advanced Accelerator Applications), which was approved by the FDA in January 2018 and consists of octreotide to which is attached the radioisotope 177Lu. In the phase 3 NETTER-1 trial, conducted in 229 patients with midgut NET, the estimated PFS after 20 months was 65.2% in the lutetium dotatate Lu-177 group and 10.8% in the octreotide group (N Engl J Med. 376:125-135, 2017). An interim analysis suggested a longer overall survival; there were 14 deaths among patients taking the novel drug, compared with 26 deaths among patients taking octreotide. A follow-up analysis showed important quality-of-life benefits, with a longer period of overall better health (28.8 months vs 6.1 months) and better physical functioning (25.2 months vs 11.5 months) (J Clin Oncol. 2018;36:2578-2584).
- Trastuzumab for uterine serous carcinoma. This is a rare form of endometrial cancer that accounts for about 10% of cases but around 40% of recurrences and deaths. About 30% of uterine serous carcinomas overexpress the HER2 gene. The HER2-targeted agent trastuzumab (Herceptin, Roche/Genentech) is used primarily for women with HER2-positive breast cancer. In a phase 2 trial involving 61 women with advanced or recurrent uterine serous carcinoma who overexpressed HER2/neu, patients were treated with either chemotherapy (carboplatin plus paclitaxel) alone or with chemotherapy plus trastuzumab. The addition of trastuzumab improved the median time to disease progression to 12.6 months, compared to 8 months for patients who received chemotherapy alone (J Clin Oncol. 2018;36:2044-2051).
- Pexidartinib for tenosynovial giant cell tumors. These rare, debilitating tumors, which are generally found in younger, working-age adults, frequently affect physical ability and cause severe loss of function. There is no standard therapy; the usual management is surgery, with multiple synovectomies and even joint replacements. The novel drug pexidartinib (Daiichi Sankyo) acts as an inhibitor of colony stimulating factor–1 receptor and has shown some promise, although it is not currently available. In the ENLIVEN trial, which involved 122 patients, the drug yielded an overall response rate of 39%, compared with 0% for patients receiving placebo. Patients taking pexidartinib showed a statistically significant improvement in pain scores, range of motion, and physical function compared to those taking placebo (J Clin Oncol. 2018;36_suppl.11502). However, serious liver toxicity occurred in some patients, and a study is underway to examine these safety concerns, the ASCO report notes.
The report also highlights a number of other clinical cancer advances, including progress made in the past year with immunotherapy in various cancer types, as well as a promising potential cancer diagnostic tests, dubbed CANCERSEEK. Most of the advances highlighted in the report have been reported in detail by Medscape Medical News.
NO USE OF PANCREATIC CANCER SCREENING
The US Preventive Services Task Force (Task Force) has the nixed the idea of screening for pancreatic cancer.
In a new draft recommendation statement and evidence review, the Task Force has given a D recommendation to pancreatic cancer screening. This means that for adults who are not at high risk for this disease and who do not show any signs or symptoms, the Task Force recommends against screening.
The new draft recommendation is an update of the 2004 final recommendation on the same topic and is consistent with the earlier recommendation.
"Although pancreatic cancer is rare, it is a devastating disease with low survival rates," said Task Force member Chyke Doubeni, MD, MPH, who is also the Harrison McCrea Dickson, MD, and Clifford C. Baker, MD, Presidential Professor and an associate professor of epidemiology at the University of Pennsylvania School of Medicine, Philadelphia.
"Unfortunately, we do not currently have an effective test to screen for pancreatic cancer," Doubeni noted in a statement.
Screening for pancreatic cancer in the general population is not recommended by any major medical organization. The American College of Gastroenterology conditionally recommends surveillance for certain high-risk individuals and suggests that surveillance be performed in experienced centers, ideally under research conditions.
The draft recommendation statement and draft evidence review on screening for pancreatic cancer will be posted for public comment on the Task Force Web site. Public comments will be accepted through March 4.
No Evidence for Accuracy of Screening Tests
Although a relatively uncommon cancer, pancreatic cancer is the third most common cause of cancer death in the United States, with an overall 5-year survival rate of 8.5%. The intervention most likely to improve survival is surgery, but only for patients with early-stage disease whose tumor is amenable to surgical resection. Even then, the median survival for early, stage I pancreatic cancer is 36 months, according to the Task Force.
Currently, most cases of pancreatic cancer are detected at an advanced stage when surgical resection is not likely to be beneficial, so there has been growing interest in identifying methods of early detection. However, the Task Force did not find any evidence that screening for pancreatic cancer improves patient outcomes.
In their review, the Task Force did not find any evidence for the accuracy of imaging-based screening tests, including CT, MRI, or endoscopic ultrasonography (EUS), for pancreatic cancer. There were no studies available that reported on the sensitivity or specificity of CT, MRI, or EUS as screening tests for this disease.
The Task Force also found no evidence that screening for pancreatic cancer or treatment of screen-detected pancreatic cancer improves any related outcomes, including disease-specific morbidity, disease-specific mortality, or all-cause mortality.
The Task Force notes that, owing to the low incidence of pancreatic cancer in the general population, the uncertain accuracy of screening tests, and the poor prognosis for patients with pancreatic cancer even when treated at an early stage, they "found adequate evidence to bound the benefits of screening for pancreatic cancer in asymptomatic adults as no greater than small."
Harms vs Benefit
The Task Force found that there was adequate indirect evidence to "bound the magnitude of the harms of screening for pancreatic cancer and treatment of screen-detected pancreatic cancer as at least moderate," based on the potential harms that could occur from false positive results and the harms of treatment. For example, some tests are invasive and can lead to complications, such as pancreatitis. Thus, the Task Force reaffirmed the 2004 conclusion that the potential benefits of screening asymptomatic adults at normal risk do not outweigh the potential harms.
"New effective screening tests are needed that can find pancreatic cancer earlier," said Task Force member Chien-Wen Tseng, MD, MPH, MSEE, a professor and the associate research director in the Department of Family Medicine and Community Health at the University of Hawaii John A. Burns School of Medicine, Honolulu, in a release. "We also need better treatments that can lead to improved survival or a cure with fewer harms."
FECAL TRANSPANT FOR OBESITY?
Could fecal microbiota transplantation (FMT), whereby medical-processed stool material from a healthy donor is introduced into the intestine of a patient with obesity, be one of the answers to tackling this disease?
That is the belief of several groups of researchers who are currently studying the potential of this novel approach.
Fecal transplants have initially proven successful in tackling life-threatening Clostridium difficile infection, and it is also being investigated as a possible treatment for ulcerative colitis, irritable bowel syndrome, and recurrent urinary tract infections.
The theory is that a "healthy" microbiome in the stool will have beneficial effects, and so fecal transplantation is also being tested as a strategy in a number of animal and human studies for the treatment of obesity and metabolic disease. A review published in 2016 (J Biol Med. 2016;89:383-388) concluded that it is "an exciting therapy with abundant potential" but for which "there has been a lack of controlled, randomized trials."
Amir Zarrinpar, MD, PhD, assistant professor, Division of Gastroenterology, University of California San Diego, La Jolla, who coauthored the 2016 review, told Medscape Medical News that there have been several more studies published in the interim, but with less than convincing results.
"That's not to say that this has put the nail in the coffin," he added, pointing out that there are currently around 20 studies registered on ClinicalTrials.gov examining the potential of fecal microbiota transplants for the treatment of obesity, metabolic syndrome, or type 2 diabetes.
Results of Double-Blind Trial Expected in May
Some of the most keenly awaited results are those from a randomized, double-blind, placebo-controlled clinical trial, which started in June 2016, by Elaine W. Yu, MD, and colleagues from Harvard Medical School, Boston, Massachusetts.
For the study, 24 individuals aged 25-60 years with a body mass index (BMI) ≥ 30 kg/m2 were randomized to fecal microbiota transplant (frozen stool) capsules from lean, metabolically healthy donors or placebo capsules for 24 weeks.
Changes in weight, insulin sensitivity, and body composition were assessed from baseline to 12 weeks, and the impact of the intervention on the intestinal microbiome of the recipient is being determined using fecal samples.
Yu told Medscape Medical News that the trial is now complete and they are analyzing the results, with the hope they will be able to present them at Digestive Disease Week this May.
Yu agreed with Zarrinpar that "there has been really a paucity of data" on this approach in humans, and noted that "there continues to be a lot of activity in animal research that connects the microbiome with obesity and the metabolic syndrome."
And she pointed out that the two randomized clinical trials with fecal microbiota transplants published so far were from the same group, led by Max Nieuwdorp, MD, PhD, professor of internal medicine, University of Amsterdam, the Netherlands.
Although they found that endoscopic delivery of fecal microbiota transplant achieved small changes in insulin sensitivity, Yu described them as "proof of principle," adding, "this clearly needs to be verified with other groups and with other styles of administration of fecal microbiota transplant."
Indeed, another clinical trial has just begun in Wales, where clinicians are delivering fecal transplants endoscopically from healthy donors to obese individuals with type 2 diabetes, following on from Nieuwdorp's work.
The study will involve people aged 18-70 years diagnosed with type 2 diabetes in the last 2 years with a BMI between 30 and 40 kg/m2.
Dean Harris, a colorectal surgeon at Singleton Hospital in Swansea, is one of the trial leaders.
"We know in patients with diabetes and obesity that their gut organisms are over-efficient in extracting energy from their food, so you have a greater number of calories absorbed from a given meal than somebody who doesn't have those conditions," he told Wales Online.
"If we can change the bacteria and organisms in the gut...we're hoping weight loss can be seen and also tighter control of diabetes."
Fecal Transplant Capsules a Better Mode of Delivery?
An issue that still needs to be addressed when trying to alter the microbiome through fecal transplant is to figure out how often it needs to be done, as it is known that the intestinal microbiome reverts to its former composition after a few weeks.
Zarrinpar made the point that "we have our microbiome for a reason," saying that it is "specific to our genetics, what our parents have passed on to us, what we eat, where we live, who we live with."
To change the microbiome without completely changing one's diet, he believes it is probably necessary to maintain a constant "pressure" on the microbiome to change using fecal transplant.
Being able to do this, Yu pointed out, "depends on how you administer the fecal transplants, and that can vary from study to study, and from group to group."
She said: "The most prevalent way of administering fecal transplants is...endoscopic delivery, which just by its more invasive nature is something that is not done frequently."
As fecal microbiota transplant capsules are an oral treatment, "it's something that can be administered potentially more frequently, more than just once or twice," she noted.
"By bringing other techniques to delivery of fecal transplants we may have the ability to modulate the microbiome on a more consistent basis and for longer periods of time."
If fecal transplants are indeed shown to be effective in tackling obesity and metabolic disease, Zarrinpar believes that it will be easier to obtain approval for a capsule version of fecal transplants because the very nature of the process would get rid of a lot of the "variability" seen with endoscopically delivered transplants.
Will Physicians and Patients Be Keen to Use Fetal Microbiota Transplants?
However, Zarrinpar doubts whether fecal microbiota transplant capsules would end up being used much, even if they do become an approved treatment.
"We would all like to think that something like this would be beneficial. On the one hand, we all know people who will do anything to lose weight, even go on crazy diets that are unsustainable...but at the same time we...already have a whole bunch of anti-obesity drugs that physicians tend not to use," he said.
He added: "There are a group of people, a certain population, that will do it but there's a lot of miseducation about obesity both in the medical field and [among] the public."
"People think that the treatment they take should revert them back to a normal BMI, whereas, besides bariatric surgery, there's nothing we have that can really do that on a regular basis on a population level."
Yu, on the other hand, had a different experience when she ran the randomized controlled trial, finding that people were very keen to take part.
"Clearly we have a lot of self-selection in those people who contact us about wanting to participate in this study, who have already gotten over the 'yuck factor' of swallowing capsules of essentially frozen stool," she said.
"But I've been doing clinical research for quite some time and I have to say that...it’s a bit like pulling teeth trying to get people to enroll.
"This is the one project where I've actually had people knocking on my door asking me to participate because there's so much interest on the lay public side about this potential question," she said.
Adding a note of caution, however, she continued: "I want to say that the excitement and enthusiasm from the lay public have, at this point, outstripped our hard data and science on this topic."
"We don't know yet whether or not this will be effective, and there are some potential harms that can come with fecal microbiota transplant."
"What I get most nervous about as a clinician is when I hear of these people who are doing these do-it-yourself fecal transplants...Outside of a monitored clinical setting, that can have potential risks that are very much unanticipated."
Some See Fecal Transplant as a More " Natural" Approach
Yu believes patients are so enthusiastic to try fecal transplants because obesity is "a notoriously difficult disease to manage and to treat."
"Anytime there is buzz about a new potential avenue to try, people are very excited [which] speaks to a level of desperation, unfortunately, amongst many patients who are looking for anything that can provide some potential help."
In addition, she thinks another reason patients are keen to try fecal transplants is that "there is already a lot of acceptance in many communities of the use of probiotics and specialized yoghurts and...going to something like fecal transplants seems less foreign to many people, and potentially more 'natural'."
Zarrinpar, in the meantime, wonders whether fecal transplants, as a microbiome-mediated treatment, will work on a population-clinical level and believes other approaches may supersede it.
"One thing that...I think could potentially have promise...is using engineered bacteria, either to express a satiety hormone or express genes that would help someone increase their metabolism."
"Those kinds of things are now being investigated in labs and could potentially have a future."
QOL AFTER TREATMENT FOR PROSTATE CANCER
Overall, men who have been treated for prostate cancer can expect quality of life equal to that as men in the general population, even those diagnosed with advanced disease, concludes the largest study of its kind.
However, the study also shows that sexual dysfunction is virtually ubiquitous among men treated for prostate cancer, regardless of age or disease stage.
In addition, men treated with androgen deprivation therapy (ADT) frequently report problems with hot flushes, low energy, and weight gain, the study shows.
"This study this study is the largest population based, patient-reported outcomes study of men with prostate cancer to date," say the authors, led by Amy Downing, PhD, University of Leeds, United Kingdom.
The study collected data on 35,823 men with prostate cancer, including 11,000 men living with locally advanced or metastatic disease (stage 3 or 4 disease), who are often excluded from quality-of-life studies, the authors note.
The study was published online January 31 in The Lancet Oncology.
The results are helpful to "those of us who treat prostate cancer to obtain a better understanding of how patients are coping with their disease and treatments," comments Fred Saad, MD, FRCSC, University of Montreal Hospital Center in Canada, in an accompanying editorial.
"Balancing the side-effects of treatment and the risks related to the cancer itself has become a priority in the field of prostate cancer," he observes.
"For the time being, we can be reassured...that in terms of patient-perceived quality of life, we might be doing a better job than we previously thought," he adds.
Study Details
The participants in this British study were initially identified through cancer registration data, and were then mailed a health-related quality of life (HRQOL) survey. The questionnaire was completed at 18 to 24 months after diagnosis of prostate cancer. The median age was 71 years and the majority of participants reported having at least one other long-term health problem.
Disease stage was known for 85.8% of the cohort out, of which 63.8% had stage I or II prostate cancer, 23.4% had stage III disease, and 12.8% had stage IV disease.
To assess functional outcomes, the EPIC-26 (Expanded Prostate Cancer Index Composite short form) was used to measure urinary incontinence; urinary irritation and obstruction; and bowel, sexual, vitality, and hormonal function, whereas the EuroQoL (EQ-5D-5L) questionnaire was used to assess measures of mobility; self-care; the ability to carry out usual activities of daily living; pain or discomfort; and anxiety or depression. The EQ-5D-5L was also used to rate a patient's self-assessed health based on how good he or she felt on the day the survey was completed.
"Mean adjusted EPIC-26 domain scores were high in all men, indicating good function, except for sexual function, for which scores were much lower," Downing and colleagues report.
Urinary and bowel function as assessed by the same questionnaire was similar across all stages of disease.
In contrast, men with stage 3 and 4 prostate cancer had significantly lower scores for vitality as well as hormonal and sexual function compared with men who had localized disease, study authors notePerhaps not surprisingly, more men who had their cancer removed surgically reported urinary incontinence than men who did not undergo prostatectomy, while men receiving ADT had worse hormonal and sexual function than men not treated with ADT, investigators add.
Poor Urinary Function
Among men reporting poor urinary function, "the need to urinate frequently was the most common urinary symptom," the researchers note.
There was little difference in the incidence of this side-effect between different stages of the disease, but there was a difference between the different treatments that were administered.
Almost one third of men in the surgical group reported having to use pads one or more times per day, a higher rate than that reported by men treated with other modalities, the researchers observe.
Importantly, bowel dysfunction was a relatively infrequent complaint, and again differed little by disease stage.
However, of those men who did report bowel problems, more men who underwent external beam radiation therapy alone (or in combination with other modalities) were more affected by bowel urgency than men who were treated with surgery alone, the researchers point out.
Problems with low energy, hot flushes, and weight gain were, in contrast, more related to stage of disease than either urinary or bowel problems, but rates of these complaints varied considerably depending on how the prostate cancer had been treated.
For example, men who had received ADT — either alone or in combination with other therapies — were much more likely to report problems with hormonal function and fatigue than men who had not received ADT.
Specifically, over 30% of men receiving ADT reported experiencing significant hot flushes, and a similar percentage of men reported having low energy; these rates were much higher than in men who had not received ADT.
Men treated with ADT were also more likely to report weight gain compared with men who had not received ADT, the investigators also point out.
Poor Erections Extremely Common
Poor or very poor erections were, on the other hand, an extremely common complaint across the whole population, being reported by 81.5% of the group, with equal percentages reporting poor or very poor overall sexual function.
Sexual dysfunction rates were high even among men with localized disease, although they were higher still among men with later stages of prostate cancer.
Men who reported undergoing active surveillance were the least likely to report poor or very poor overall sexual function, yet even in this group, over half of patients registered this as a major complaint.
About 45% of men overall also indicated that they were bothered by their poor sexual function, although this complaint decreased slightly with age.
About 40% of men who reported poor or very poor sexual function noted that they were offered medications, devices, or counseling to help improve their sex lives.
However, this meant that close to 56% of the group were not offered any intervention for sexual dysfunction, and this percentage remained relatively high even in the youngest cohort.
Of those who were offered some sort of intervention, over 37% didn't bother with any of them and almost one quarter of recipients indicated that the intervention did not help.
Pain or discomfort were relatively common in the overall group, being reported by close to 42% of the men surveyed.
However, men with stage 4 prostate cancer were the most likely to report pain or discomfort, as well as difficulties carrying out activities of daily living.
That said, "the overall mean adjusted self-assessed health score was 76.3," Downing and colleagues report, which was only 5.7 points lower in men with stage 4 disease compared with men with stage 1 or 2 prostate cancer.
ADT and Quality of Life
As the authors note, most men with stage 3 or 4 prostate cancer in this particular cohort were on long-term or indefinite ADT.
Given the detrimental effects of ADT on both vitality and hormonal function, "results suggest that clinicians should pursue treatment approaches that preserve testosterone function when possible and minimize ADT use," the authors advise.
Steps to mitigate side effects associated with ADT include the use of intermittent rather than continuous ADT; avoidance of the unnecessary use of ADT; and a reduction in the duration of treatment from 3 years to 1 year.
Predictive Markers
In the editorial, Saad expresses the hope that, eventually, the medical community will have predictive markers to help them discriminate between aggressive prostate cancer in need of early aggressive therapy and the more indolent forms of the disease.
Other biomarkers may also allow physicians to identify men who are most likely to suffer the ill effects from ADT.
"Until then, we must re-establish some reasonable equilibrium and return the focus to the fact that, first and foremost, we are treating a life-threatening disease for most of those diagnosed," Saad emphasizes.
In the recent past, aggressive screening for, and treatment of, prostate cancer probably did "more harm than good in many cases," Saad comments.
He admits that issues of quality of life, and this worry about doing more harm than good, has led him to "delay interventions in cancers [that] I underestimated," he writes, and he humbly regrets having delayed treatment decisions.
However, while aggressive screening and indiscriminate treatment is no longer acceptable, "the worry now is that the pendulum might have swung in the opposite direction and we fear that we might slowly creep back to the era in which most patients were treated at a late or incurable stage," he suggests.
"Clearly, continued intensive efforts in research are needed to achieve the aim of optimising and personalising care of patients with prostate cancer," he concludes.
The study was funded by the Movember Foundation in partnership with Prostate Cancer UK. Downing and Saad have disclosed no relevant financial relationships; Downing's study coauthors have disclosed relevant financial relationships, which are listed in the published manuscript.
Subscribe to:
Posts (Atom)


























































